I-SAFE
De-risk TCR therapies before the clinic
Comprehensive characterization of TCR reactivity against the whole human proteome.
Unexpected off-target and cross-reactive TCR recognition remains one of the greatest risks in TCR-based immunotherapies. I-SAFE provides a comprehensive, proteome-wide approach to safety screening—helping teams identify risk early and advance safer candidates with confidence.

The Safety Challenge
Why TCR Safety Requires a Proteome-Wide View
T cell receptors are exquisitely sensitive, capable of recognizing subtle molecular differences between peptides. While this precision enables powerful anti-tumor responses, it also creates risk: even low-level or unexpected antigen recognition can lead to severe toxicity.
Traditional safety approaches focus on limited tissue panels or predicted targets, leaving blind spots across the human proteome. Without unbiased, comprehensive screening, dangerous off-target interactions may go undetected until late in development—or the clinic.
I-SAFE Platform Capabilities
Human Proteome–Wide TCR Safety Screening
I-SAFE functionally screens therapeutic TCRs against a synthetic library representing the entire human proteome. This proteome-wide approach enables early detection of off-target and cross-reactive interactions across thousands of biologically relevant peptides.
Screen TCRs against comprehensive, genome-scale peptide libraries
Detect rare and unexpected off-target interactions missed by traditional assays
Inform go/no-go decisions with a robust preclinical safety benchmark
How I-SAFE Works
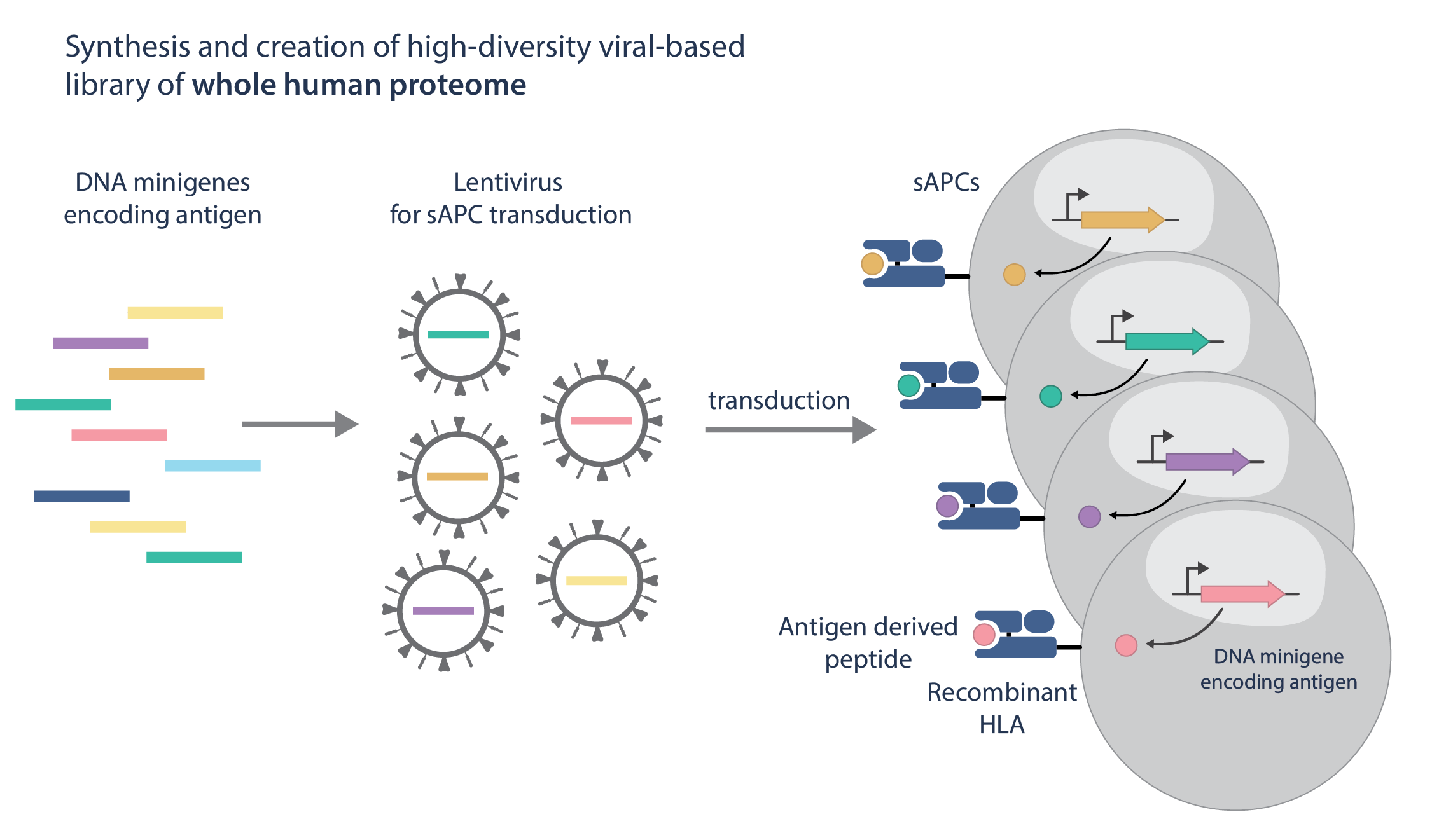
Synthetic APC Libraries Built from the Entire Human Genome
I-SAFE leverages overlapping, tiled minigene libraries that encode the human reference proteome and are expressed in synthetic antigen-presenting cells (APCs). This design enables functional, cell-based assessment of TCR reactivity at genome scale.
Tiled minigene strategies capture self-antigens and rare peptides
Functional readouts reflect biologically relevant T cell activation
Cell-based screening provides actionable insight beyond in silico predictions
Flexible, Reusable, and Strategic Safety Screening
Designed for Reuse, Customization, and Smarter Risk Assessment
I-SAFE libraries are built for reuse across programs and adaptable to multiple HLA contexts, enabling consistent and scalable safety screening. For deeper insight, custom SNP-based libraries support personalized off-target profiling.
Rather than relying on assumptions about tissue-specific expression, I-SAFE enables unbiased proteome-wide screening first—providing the data needed to rationally design targeted tissue studies tailored to each TCR.
Partner with Us
Advance your TCR programs with confidence by identifying safety risks before they become clinical liabilities.